-40%
ECG1212G Digital Touch 12 channel ECG Machine EKG Electrocardiograph Printer New
$ 316.27
- Description
- Size Guide
Description
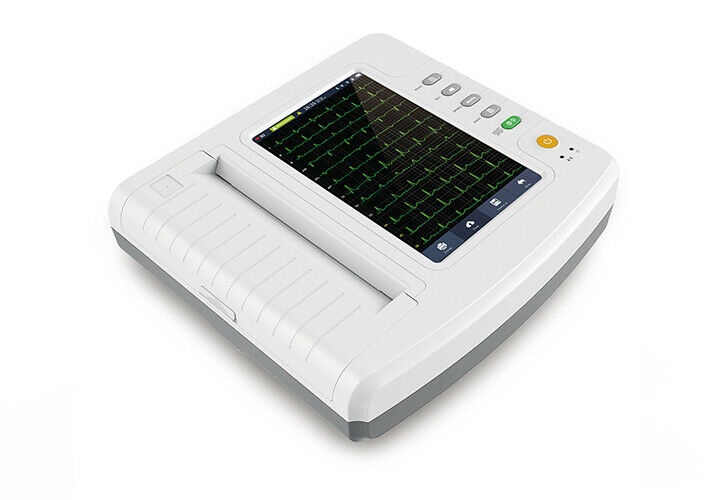
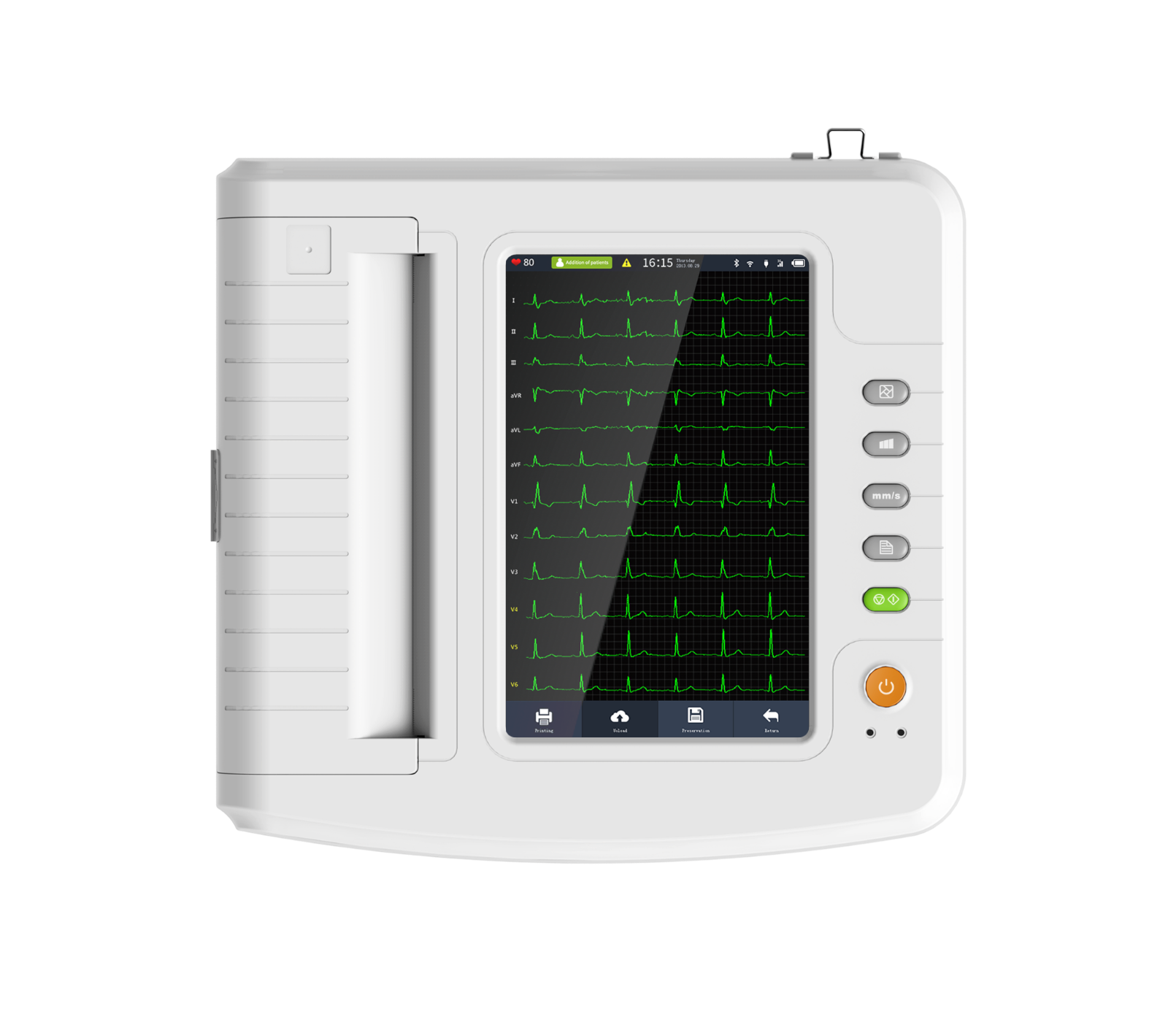
Test ItemECG1212G Touch 12 Channel ECG Machine
Description
Introduction
Introduction
The device is an electrocardiograph which can collect 12-lead ECG signal simultaneously and print ECG waveform with thermal printing system. It features in, recording and displaying ECG waveform in auto / manual mode; measuring, analyzing and diagnosing ECG waveform parameters automatically; prompting for “Lead off” and “Lack of paper”; optional interface language (Chinese / English, etc.); built-in lithium battery, AC / DC; selecting rhythm lead freely to observe abnormal heart rhythm; case database management.
Functions
1)Adopt 10.1'' high-resolution color LCD, operate by full-touch screen and function bu ttons, convenient to use.
2)Sync collection for 12-lead ECG, support 12-lead and Cabrera-lead waveform display. Get high-quality ECG waveform via power frequency filter (50 / 60 Hz), baseline filter and EMG filter (25 / 35 Hz) of ECG signal.
3)Display 3 / 6 / 12 - lead ECG waveform, HR value, print mode, sensitivity, paper speed, filter state, clock, battery power, background grid, measured parameter and interpretation, etc. on one screen, and with prompt functions for lead - off, overload and system working state.
4)Power supply: AC / DC (which can adapt AC 50 / 60Hz), with built-in rechargeable lithium battery, charging circuit, perfect over-current and over-voltage protection circuit.
5)In optimal DC state, up to 10-hour standby time, continuous print for more than 3-hour, which meets the requirements of visiting a patient at home and body examination.
6)Built-in thermal printer, support multiple printing modes and formats, such as, auto M × N, M × N + 1, M × N + 2, rhythm M line and manual, etc. The contents including time, paper speed, sensitivity, calibration signal, lead name, filter state and patient information can be printed. The waveform length printed, measurement parameter output, diagnosis conclusion, superimposed QRS waveform, histogram, trend chart and interval list, etc. can be set. With the print functions (timing print and auto-print for arrhythmia, etc.), which meet different requirements.
7)With the functions of auto-measurement and auto-interpretation for routine ECG parameters, provide measured results and auto-diagnosis conclusions for HR, PR interval, P Duration, QRS Duration, QT / QTc interval, P / QRS / T Axis, R (V5), S (V1), R (V5) + S (V1) amplitude, etc. which reduces the doctor’s burden.
8)Built-in memory stores 4000 cases (data saving time: 10 s) at least, convenient for case review and statistic.
9)Historical cases can be reviewed, inquired, modified, transmitted, printed, lead corrected, the cases can also be exported to other electronic file formats (dat, pdf, xml, bmp, jpeg, etc.)
10)Chinese / English operating interface, the report in corresponding language can be printed; built-in virtual keyboard, support Chinese / English input method.
11)Support external USB standard keyboard, mouse, scanner and printer.
Performance
1)Input mode: floating and defibrillation protection
2)Lead: standard 12-lead
3)Patient leak current: < 10 µA
4)Input impedance: ≥ 2.5 MΩ
5)Frequency response:
Nominal input amplitude Input frequency and waveform Relative output response
1.0 0.67 Hz ~ 40 Hz, sinusoidal ±10 %a
0.5 40 Hz ~ 100 Hz, sinusoidal +10 %, -30 %a
0.25 100 Hz ~ 150 Hz, sinusoidal +10 %, -30 %a
0.5 150 Hz ~ 500 Hz, sinusoidal +10 %, -100 %a
1.5 ≤1 Hz ,200 ms, triangular +0 %, -10 %b
a relative to 10 Hz output b relative to 200 ms output
6)Time constant: ≥ 3.2 s
7)CMRR: > 105 dB
8)Filter: power frequency (AC 50 / 60 Hz), EMG (25 Hz / 35 Hz (-3 dB)) and baseline drift
9)Recording mode: thermal printing system
10)Paper size: 210 mm (W) × 20 m (L), high-speed thermal paper
11)Paper speed: 12.5 mm/s, 25 mm/s, 50 mm/s, error: ± 5 %
12)Gain (sensitivity): 5, 10, 20 mm / mV, accuracy: ± 2 %
Standard sensitivity: 10 mm/mV ± 0.2 mm/mV
13)Record mode: auto, rhythm and manual record
14)Measurement parameters: HR, P - R interval, P Duration, QRS Duration, T Duration, Q - T interval, Q - Tc, P Axis, QRS Axis, T Axis, R (V5) amplitude, S (V1) amplitude, R (V5) + S (V1) amplitude
15)Safety class: class I, defibrillation-proof type CF applied part
16)Polarizing voltage: ± 610 mV
17)Noise level: ≤ 12 µVp-p
18)Sampling frequency for ECG signal input: 32 kHz
19)Sampling frequency for waveform data processing: 1 kHz
20)Sampling accuracy: 24 - bit
21)Minimum detection signal: a 10 Hz, 20 µV (peak - peak value) sinusoidal signal can yield a visual recorded deflection.
22)Pacemaker detection channel: II
23)Accuracy of input signal reproduction: ± 5 %
24)Amplitude quantization: ≤ 5µV/LSB
25)Time deviation in each channel: <100 µs
26)Power supply:
AC: 100V ~ 240V (50 / 60 Hz)
DC: 14.8 V / 5200 mAh rechargeable lithium battery
27)Degree of protection against ingress of liquid: IPX0
28)Working mode: continuous operation
Accessories
Standard:
One lead cable
One limb electrode
One chest electrode
One thermal recording paper
One power cord
One earth wire
One User Manual
Physical characteristic
Operating:
a) Environment temperature: 5 ℃ ~ 40 ℃
b) Relative humidity: 25 % ~ 95 % (non - condensing)
c) Atmospheric pressure: 700 hPa ~ 1060 hPa
Transport and storage:
a) Environment temperature: - 20 ℃ ~ + 55 ℃
b) Relative humidity: ≤ 95 %
c) Atmospheric pressure: 500 hPa ~ 1060 hPa
Dimension: 340 mm (L) × 320 mm (W) × 86 mm (H)
Weight: 5 kg
Payment
1.Paypal account
Shipping
Clearance: we will ship your item to your confirmed address.
Buyers' responsibility to pay duties,taxes and other extra charges by the government in your country.
Terms of Sale
Buy safe Products
The following FDA Disclaimer is required for all eBay listing in Healthcare category and is included for REFERENCE:
The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies.If the item is subject to FDA regulation, We will verify your status as an authorized purchaser of this item before shipping of the item.
If you have questions about legal obligations regarding sales of medical devices, you should consult with the FDA's Center for Devices and Radiological Health.
The Fingertip Pulse Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG) with the code 197923, and certified by FDA of United States and CE,TUV of Europe.The Fingertip Pulse Oximeter that is FDA 510K Approved
About Us
Contec Medical Systems Co.,Ltd; 20 Years manufacturer,we have stock in USA and China.
Contact Us
Whatsapp/Wechat:+86 18716007715; Skype:contec.tessy; contecmxfATgmail.com
Only English user guide, if you need any other language, please contact us!
Only English user guide, if you need any other language, please contact us!