-40%
FDA ECG90A Touch Machine Digital 12 lead 1 Channel EKG PC Software USA Fedex
$ 131.47
- Description
- Size Guide
Description
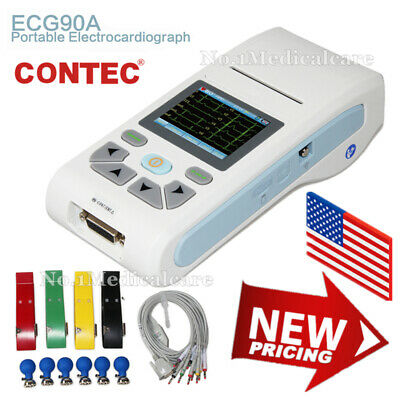
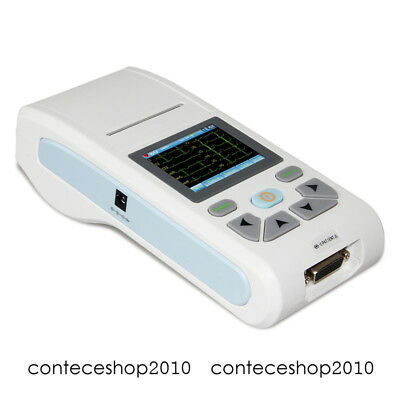
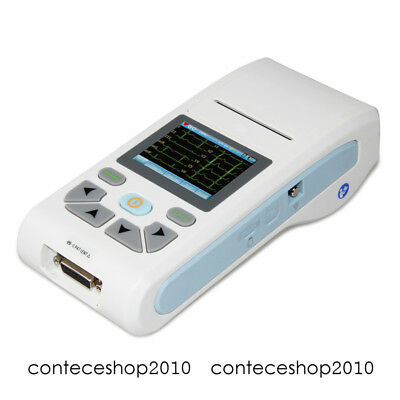
ECG90A ElectrocardiographDescription
Introduction
ECG90A is an Electrocardiograph which can collect 12-lead ECG signal and print waveform by the thermal printing system. It features in: recording and displaying ECG waveform in AUTO/Manual mode, measuring and diagnosing ECG waveform parameters automatically, prompting for "Lead off" or "Lack of paper", optional interface languages(Chinese/English), case database management.
Function
1)Sync collection for 12-lead ECG, adopt digital signal processing technology and get high-quality ECG waveform via power frequency filter, baseline filter and EMG filter of ECG signal.
2)Particular and high-accurate digital filter eliminates baseline drift, no distortion to ECG waveform, greatly enhances anti-baseline drift capacity, convenient for waveform judgment.
3)Display of 3/6/12-lead ECG, print mode, sensitivity, paper speed and filter state, etc. on one screen, convenient for contrastively diagnosing.
4)Real-time and continuously record the clear and accurate ECG waveform and annotation character(including lead mark, sensitivity, paper speed and filter state, etc.).
5)Adopt high-resolution thermal printing system(8 dot/mm), no need for any adjustments. Recording frequency: up to 150Hz.
6)With the functions of auto-analysis and auto-diagnosis for routine ECG parameters, provide measurement results and auto-diagnosis conclusion for HR, P-R interval, P Duration, QRS Duration, Q-T interval, Q-Tc, P Axis, QRS Axis, T Axis, R(V5), S(V1), R(V5)+S(V1), etc. which reduces the doctor’s burden.
7)Optional SD card stores up to 100 cases, which is convenient for doctor to review case and statistic.
8)In review mode, case waveform stored can be checked, measurement parameter and diagnosis information can be automatically analyzed.
9)Two languages(Chinese or English) interface and report.
10)Optional ECG-Sync software, connect with PC by USB2.0 interface to form a ECG workstation, collect real-time data to the software for analyzing and printing the report, and old cases stored in Electrocardiograph can be uploaded to PC.
11)Power supply: AC/DC, and in optimal DC state, built-in rechargeable lithium battery can continuously work for 4-hour, print up to 90-minute and 150-ECG, which meets the requirements of visiting a patient at home and body examination. Rechargeable lithium battery is detachable, which is convenient for user to replace and charge in any time.
Performance
Input mode: floating and defibrillation protection
Frequency response: 0.05Hz ~ 150Hz(-3dB~+0.4dB)
CMRR: >60dB, >100dB(add filter)
Time constant: ≥3.2s
Patient leak current: <10µA
Calibration voltage: 1mV
Sensitivity: 5, 10, 20 mm/mV ±5%, standard sensitivity: 10mm/mV±2%
Noise level: ≤15µVp-p
Input circuit current: ≤50nA
Input impedance: ≥50MΩ
Sampling accuracy: 12-bit
Safety classification: class I, type CF and defibrillation-proof applied part
Recording mode: thermal printing system
Measurement parameters: HR, P-R interval, P Duration, QRS Duration, T Duration, Q-T interval, Q-Tc, P Axis, QRS Axis, T Axis, R(V5), S(V1), R(V5)+S(V1)
Paper size: 50mm(W)×20m(L)
Sampling frequency: 1000Hz
Polarizing voltage: ±500mV
EMG interference filter: 25Hz/35Hz (-3dB)
Power frequency filter: AC 50Hz/60Hz(-20dB)
Paper speed:
Auto: 6.25, 12.5, 25, 50mm/s, ±5%
Manual: 6.25, 12.5, 25, 50mm/s, ±5%
Power supply: DC 12V adapter, 7.4V/2000mAh rechargeable lithium battery
Waterproof degree: IPX0
Working mode: continuous working
Accessories
Standard:
A lead cable
A limb electrode
A chest electrode
A thermal recording paper
A power cord
An earth wire
A user manual
A power adapter
ECG-Sync software+USB cable
ECG electrode
SD card
Physical characteristic
Working environment
Temperature:
5℃~40℃
Relative humidity: 25%~95%(non-condensing)
Atmospheric pressure: 700hPa~1060hPa
Transport and storage environment
Temperature:
-40℃~+55℃
Relative humidity: ≤95%
Atmospheric pressure: 500hPa~1060hPa
Dimension: 207mm(L) × 96mm(W) × 62mm(H)
Weigh: 0.5Kg
Buy Safe Products
The following FDA Disclaimer is required for all eBay listing in Healthcare category and is included for REFERENCE:
The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If the item is subject to FDA regulation, We will verify your status as an authorized purchaser of this item before shipping of the item.
The item is registered on the Australian Register of Therapeutic Goods(ARTG) with the code 197923,
and certified by FDA of United States and CE,TUV of Europe. The Fingertip Pulse Oximeter that is FDA 510K Approved.
Payment
We accept payment by PayPal. PayPal enables you to make payment quickly and securely online.
1: We accept payment via PayPal.
2: Please pay the money within 5 days after the auction ended.
3: Please offer home or mobile phone when the item's Value exceeds 100 (USD, EUR, AUD, GBP) so that we can
make sure you get the item safely.
Shipping
1. Items are dispatched with in 48 hours after payment receiced or eCheck cleared.
2.Please make sure the address and tel number are right,both of the information is very important for delivery,thank you for your kindly cooperation.
3.After the shipment,we will add the tracking number on both ebay and paypal website,you can check it and track it by the number.
4. Usually we ship this item from China by air small package,it takes about 10-45 days arrive,and we will supply a tracking number to check its state online.
Terms of Sale
We offer 60 days return policy. 100% satisfaction is our goal!
1. All items are brand new, with 2 years warranty.
2.Client is reaponsible for the custom cleatance and custom duty.
If any, we will not be responisble for the customs clearance and duty, if you have special requirments for the declaration for your customs office, please contact us before your payment.
3. Customer is responsible for the returning shipping fee and we will send the replacement after receiving the returned item.
4. Your positive feedback will be appreciated very much, and in any case, please do contact us before leaving nagative feedback or ship the item back, we promise we will give you a satisfied answer.
About Us
Contec Medical Systems focusing on research, manufacture and distribution of medical instruments,was founded in 1992
as a high-tech company.
At present there are more than 1200 employees in our company.Our product line covers a wide range of 13 categories.
Most of the domestic hospitals are our customers.
Contec hopes to cooperate with international companies to supply more innovative design and advanced technology products
We sincerely welcome you to become one of our global partners.We are looking forward to establishing a successful business relationship with you.
Powered by SoldEazy
We only have the English Manual in the standard package, If you need any other language Manual, please contact us freely