-40%
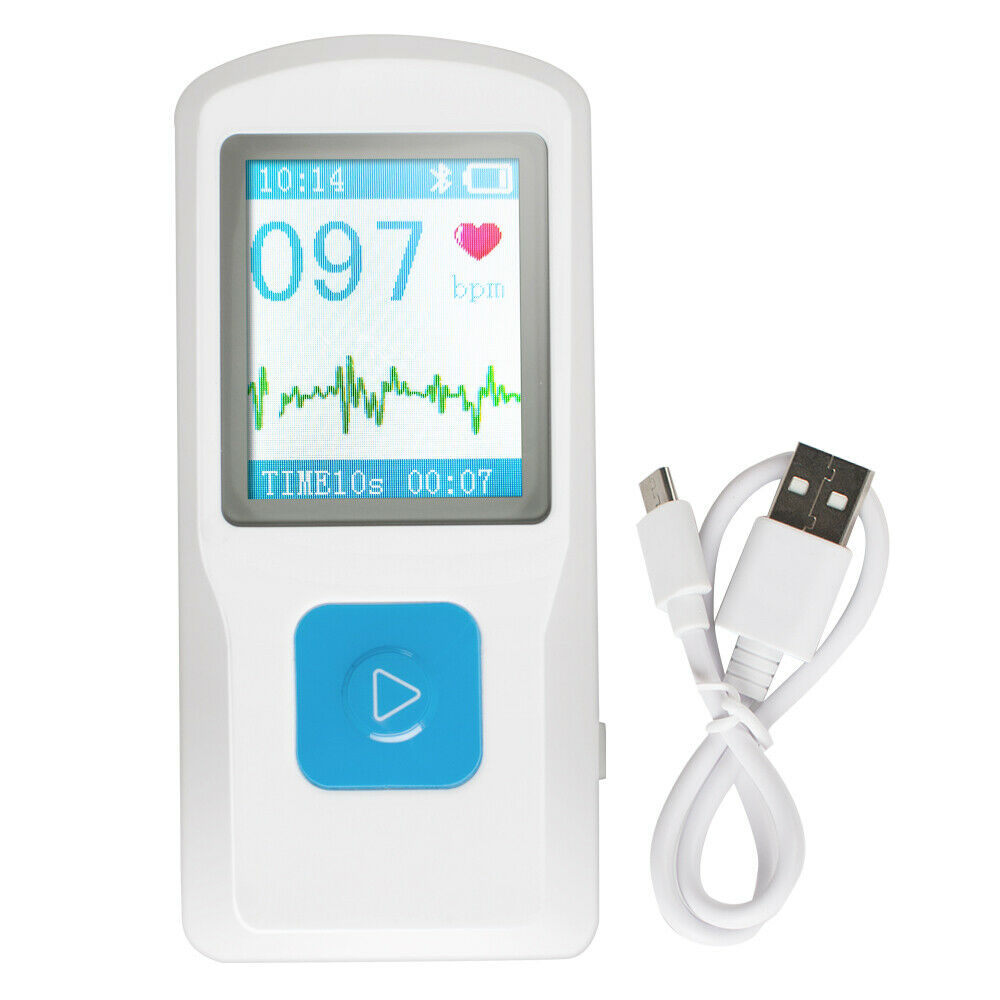
Handheld Mini ECG Machine EKG Machine EKG Monitor Heart Beat Monitor USB PM10
$ 26.13
- Description
- Size Guide
Description
Model: 600051-00155Introduction
PM10 Portable ECG Monitor is a device for checking ECG,which is applicable for family and
individual user, it is a good helper to early prevent from cardiovascular diseases and reduce risks.
Intelligent design, achieves remote health management by using with mobile application, it can
automatically start measurement, store ECG, upload data, download health conclusion and obtain
doctor advice at the first moment.
Main Features
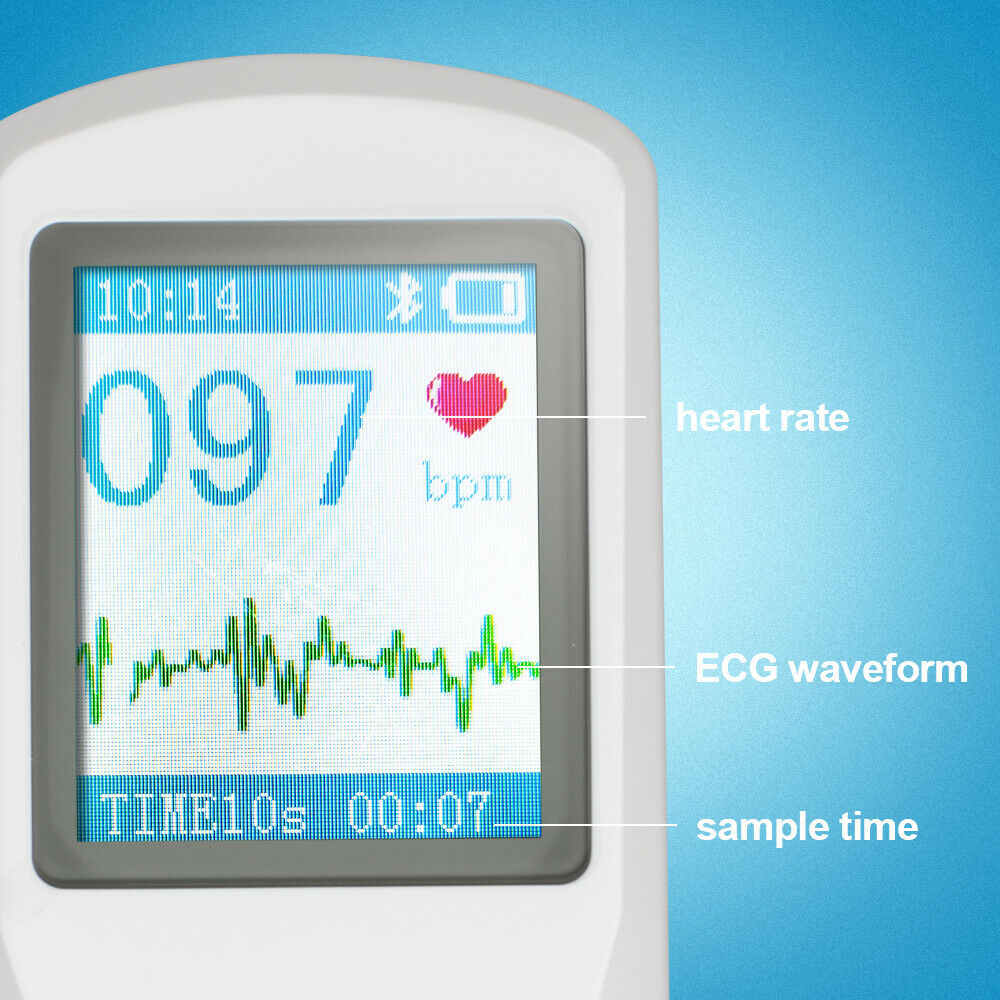
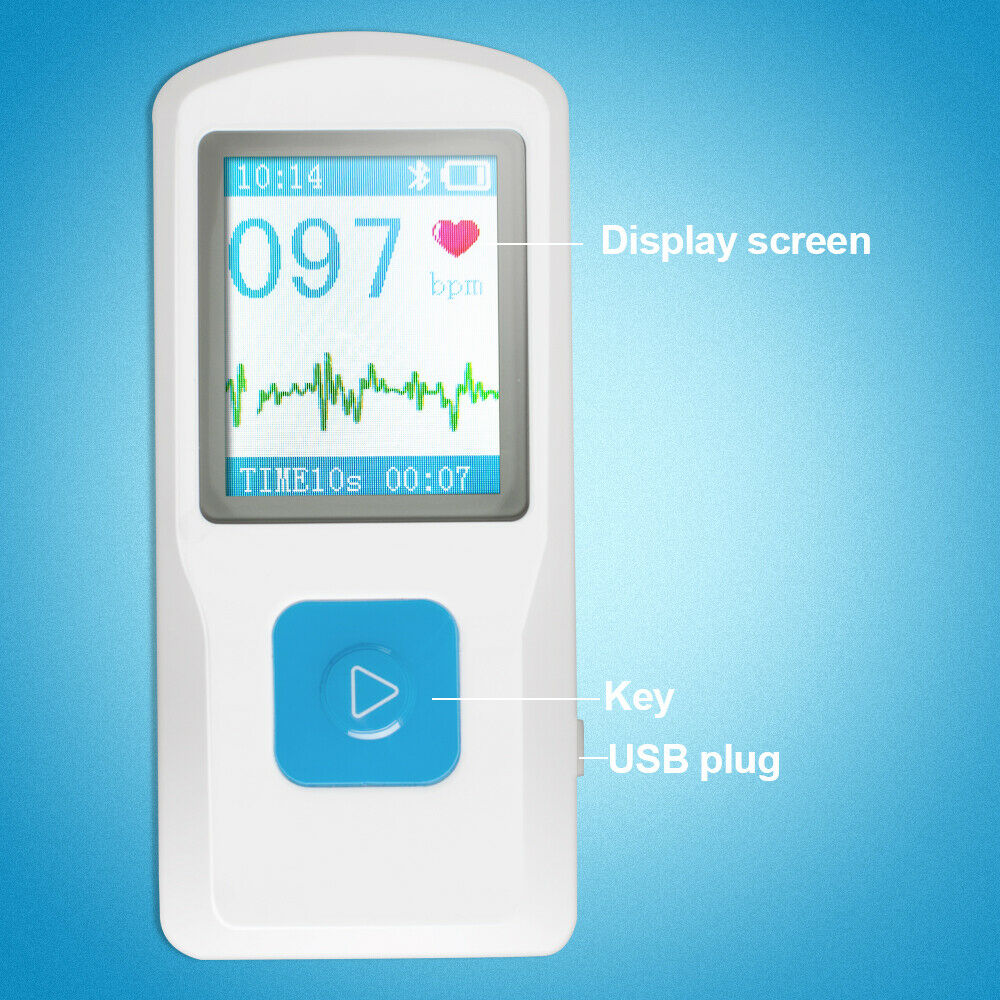
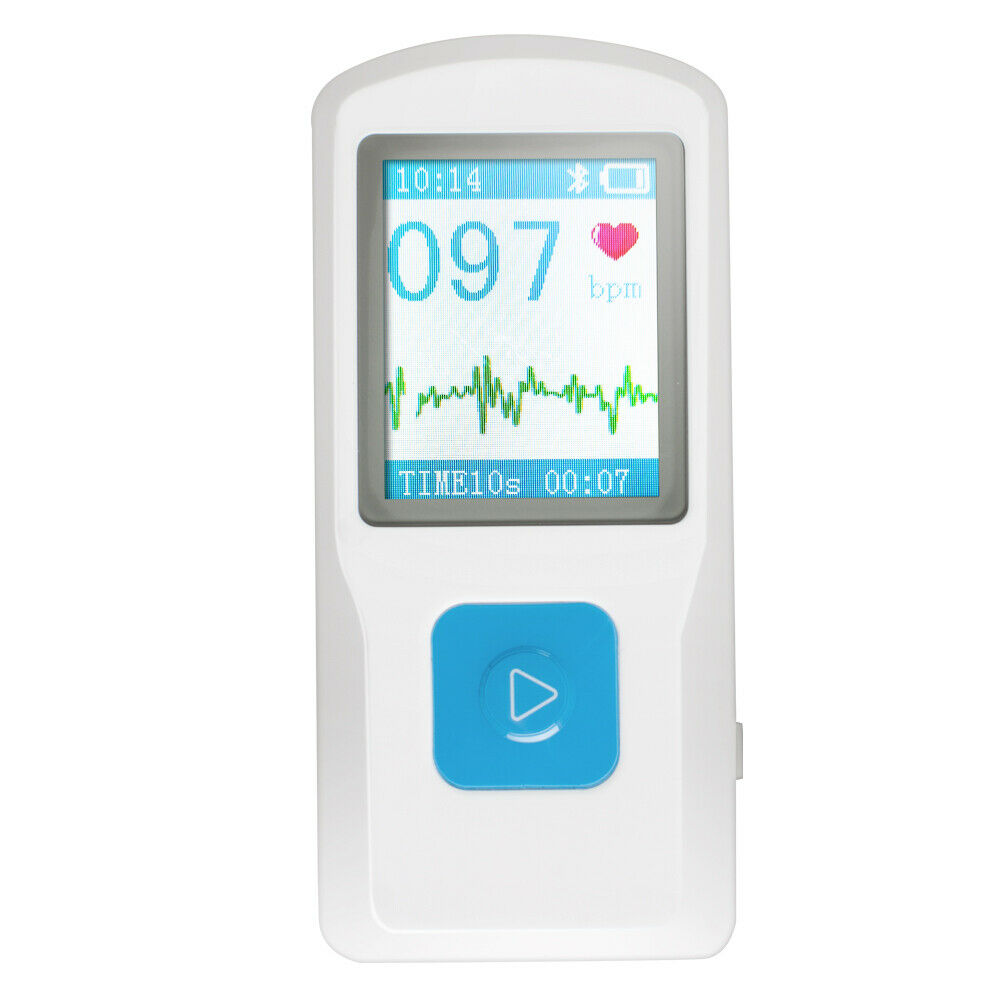
1.77" color TFT-LCD.
Quickly to check ECG once finger touching, convenient to operate.
Accurate conclusion can be obtained immediately after measuring.
The built -in rechargeable lithium battery can continuously record for up to 500 cases in full charging.
Bluetooth transmission.
ECG data can be saved to CLOUD Platform permanently, convenient to check and analyze.
Historical data can be printed at any time, convenient to diagnose.
Physical Characteristic:
Battery Voltage:DC3.7V
Operating Current:≦150mA
Dimension: 100mm(L)×45mm(W)×15mm(H)
Weight:about 60g
Package list:
1×Main Machine
1×USB Line
FDA declaration :
Statement:The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If so, do not bid on this item unless you are an authorized purchaser.If the item is subject to FDA regulation, we will verify your status as an authorized purchaser of this item before shipping of the item.(Seller Name: Christine;Country: China;City: Beijing;TEL.:86-15210656055).
This item has been cleaned and treated according to the manufacturer's instructions.
The Fingertip Pulse Oximeter is certified with the US FDA 510K No. K070371, the CE & TUV of Europe and it is on the Australian Register of Therapeutic Goods (ARTG) with the code 136606.
The Powered Surgical Instrument / Speed 808 System is certified with the US FDA 510(k) Number:K132989
The Powered Surgical Instrument / Hair Remove Device is certified with the US FDA 510(k) Number:K180353
The Powered Surgical Instrument / Hair Remove System is certified with the US FDA 510(k) Number:K141973
massager, vacuum, light induced heating / Slimming Treatment Device is certified with the US FDA 510(k) Number:K161892
Contact Us
Any questions, please contact us through eBay message, We will reply within 1-2 business days (public holidays not included).
working time: Monday - Friday 9:00 AM - 5:30PM (Beijing)