-40%
New 3Channel 24 hours Recorder ECG/EKG Holter Monitor System FDA CONTEC US
$ 157.87
- Description
- Size Guide
Description
2020 New 3 Channel 24 hours Recorder ECG/EKG Holter Monitor System FDA CONTEC USIntroduction
Dynamic ECG Systems adopt 3-lead, which can continuously record ECG waveform for 24-hour and analyze ECG waveform by the PC software. It is applicable for use in medical institution and community.
Function
1)The recorder can real-time store ECG data for 24-hour.
2)Arrhythmia analysis basing on MCSSTM and TMCATM can greatly reduce doctor’s work.
3)With more than 10 templates(such as atrial premature beat, ventricular premature beat, long interval, atrial fibrillation, etc.) and many user-defined modules, which can almost distinguish any kind of pathologic waveforms.
4)Flexible analysis channel selection function, which ensures that any channels can be selected as the main analysis channel.
5)Flexible atrial fibrillation analysis, which ensures that physicians can use the whole or segmented AUTO/manual analysis, more accurate in atrial fibrillation analysis.
6)All pacemakers(such as AAI, VVI, DDD etc.) can be analyzed by the powerful pacemaker analysis function.
7)With many pacing analysis templates, such as “Dual chamber pacing”, “Atrial pacing”, “Ventricular pacing”, “Ventricular Pseudofusion” and “Ventricular asynchronous pacing”, etc.
8)Single or full-lead ECG in any time bucket can be reviewed by the fast review analysis function.
9)With analysis functions for 5-minute, 1-hour and 24-hour heart rate variability.
10)One-key print, convenient and fast to print the reports.
11)Perfect case management function.
12)The risk of sleep breath pause can be predicted by the unique "Sleep breath pause syndrome"analysis function.
13)The death risk in patients with myocardial infarction can be predicted by the "HRT" analysis function.
14)“T-Wave alternation” is an important index to predict the malignant arrhythmia and sudden cardiac death
Performance
Lead: 3-lead
Record time: 24-hour
Power supply: two “AA” batteries
Interface: USB2.0
Calibration voltage: 1mV±5%
Noise level: ≤30μV
CMRR: ≥60dB
Low-frequency characteristics: time constant≥3.2s
Scan speed: 25mm/s±5%
Polarizing voltage: ±300mV, sensitivity: ≤±10%
Minimum measure signal: 50 µV p-p
Accessories
Lead cable (1 set)
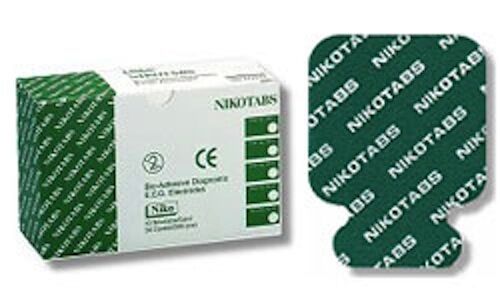
ECG electrode (1 bag)
USB cable(1)
Disk(1)
Bag(1)
User manual(1)
Physical characteristic
Dimension: 111mm(L) × 60mm(W) × 25mm(H)
Weight: about 105g(without batteries)
Payment:
*We accept payment from PAYPAL, Bank transfer,
Visa,Credit Card and others.
*All payments are expected within 7 days after the last
winning auction is closed.
Shippment
*
Clearance: we will ship your item(s) to your PayPal confirmed address.Please leave me your clear
address,telephone,postal code.
*
All items will be dispatched within 2 bussiness day.
*
To US buyer,ship from USA warehouse by USPS 2-6 working days arrival.
*
To other country buyer,ship from China warehouse by Air mail 2-4 weeks arrival
* The item will be declared as lower value for shipping only.We'd like to cooperate with you and declare the value of it
as
you want us to, so that it is more easier for you to clear the custom.
Buy safe Products
The following FDA Disclaimer is required for all eBay listing in Healthcare category and is included for
REFERENCE:
The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state
and local regulatory agencies.If the item is subject to FDA regulation, We will verify your status as an
authorized purchaser of this item before shipping of the item.
If you have questions about legal obligations regarding sales of medical devices, you should consult with
the FDA's Center for Devices and Radiological Health.
The Fingertip Pulse Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG) with
the code 197923,
and certified by FDA of United States and CE,TUV of Europe.The Fingertip Pulse
Oximeter that is FDA 510K Approved
Powered by SoldEazy
Only English user guide,if you need any other language,please contact us!