-40%
Touch screen Digital 6Channel 12 Lead Electrocardiograph ECG/EKG Machine Clinic
$ 205.39
- Description
- Size Guide
Description
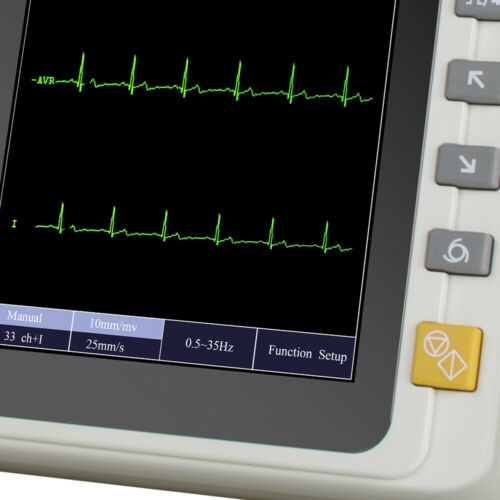
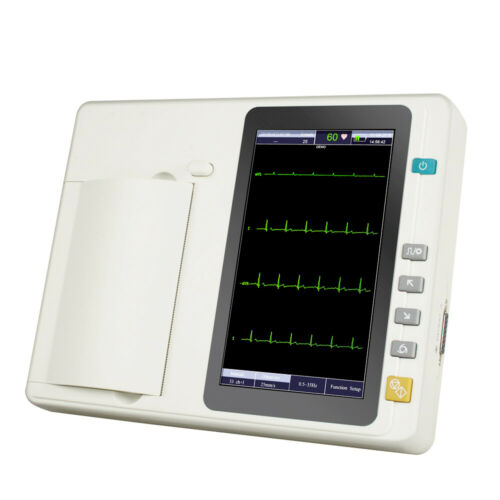
Portable 7’’ LCD Touch Screen Digital 6 Channel Electrocardiograph ECG/EKG MachineModel: M600061
Description:
Features:
7 inch graphic LCD display,touch screen and function key control at the same time, more convenient and shortcut.
Six
channel ECG machine with interpretation
Auto-regulation of baseline drift can effectively inhibit baseline drift, optimizing the printing position to achieve high-quality ECG.
With automatically measuring and analyzing function for ECG parameters to reduces the doctor’s burden and improves work efficiency.
With a high resolution thermal printer to print out ECG trace,describing the trace clear and accurate,annotation as well as related parameters for diagnostic reference.
12-lead ECG simultaneous acquisition.
Adapt 110-240V, 50/60Hz AC power supply.
Specifications:
Lead
standard 12 leads
lead acquisition
synchronously 12 leads
Input circuit
Floating; Protection circuit against Defibrillator effect
Input Impedance
≥50MΩ
Input circuit current
≤0.0.05μA
Record mode
Automatic: 3CH×4+1R, 3CH×4, 3CHx2+2CHx3,3CHx2+2CHx3+1R,6CHX2;
Manual: 3CH, 2CH, 3CH+1R, 2CH+1R;
Rhythm: Any lead selectable.
Filter
EMG Filter: 25 Hz / 30 Hz / 40Hz/75 Hz / 100 Hz / 150Hz
DFT Filter: 0.05 Hz/ 0.15 Hz
AC Filter: 50 Hz / 60Hz
CMRR
>100dB
Patient current leakage
<10μA
Input Circuit Current
<0.05µA
Frequency Response
0.05Hz~150Hz (-3dB)
Sensitivity
2.5mm/mV, 5 mm/mV, 10 mm/mV, 20 mm/mV
Anti-baseline Drift
Automatic
Time constant
≥3.2s
Noise level
<15μV
p-p
Paper speed
12.5 mm/s, 25 mm/s, 50 mm/s
Recording mode
Thermal printing system
Paper specification
110mmx20m paper roll
LCD display
7” graphic LCD
Safety classification
IEC60601-1 class I, type CF
Power supply
AC: 100~240V, 50/60Hz, 30VA~100VA
DC: 14.8V/2200mAh, built-in lithium battery
Fuse
AC220V±10% 2*Φ5×20mm,T2A/250V AC time lag
AC110V±10% 2*Φ5×20mm,T4A/250V AC time lag
Package List:
ECG Machine×1
ECG Lead Wire×1
Limp Clip×4
Chest Suction Ball×6
Thermal Printing Paper×1
Fuse×1
Power Cable
×
1
FDA Declaration:
The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If so, do not bid on this item unless you are an unauthorized purchaser.
If the item is subject to FDA regulation, We will verify your status as an authorized purchaser of this item before shipping of the item. This item has been cleaned and treated according to the manufacturer's instructions. (The seller’s name: Sarah Liu City: Beijing State: Beijing Country: China
TEL:18330168300
)
The Fingertip Pulse Oximeter is certified with the US FDA 510K No. K070371, the CE & TUV of Eureope and it is on the Australian Register of Therapeutic Goods (ARTG) with the code 136606.
The Powered Surgical Instrument / Speed 808 System is certified with the US FDA 510(k) Number
:
K132989
The Powered Surgical Instrument / Hair Remove Device is certified with the US FDA 510(k) Number
:
K180353
The Powered Surgical Instrument / Hair Remove System is certified with the US FDA 510(k) Number
:
K141973
massager, vacuum, light induced heating / Slimming Treatment Device is certified with the US FDA 510(k) Number
:
K161892
1.We only accept payment by Paypal.
2.Please make sure you PayPal account is valid prior bidding.
3.Great appreciate for prompt payment. Unpaid item will be filed without receiving payment in 7 days after listing ended.
1. After full payment is received and cleared, we will ship it ASAP
2. A complete and correct shipping address is essential.
3. Customers with shipping addresses outside of the U.S. are responsible for all duties and import taxes. Please contact your customs for information.
More than 200$ ,we usually send via international express,such as DHL,UPS ,EMS ,TNT and so on.
1. we accept returns, contact us within 14days from delivery date and we will give you a full refund or exchange.
2. All items are in brand new condition unless specified.
The following FDA Disclaimer is required.
REFERENCE:
The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and stateand local regulatory agencies. If so, do not bid on this item unless you are an authorized purchaser. If the item is subject to FDA regulation, We will verify your status as an authorized purchaser of this item before shipping of the item.
This item has been cleaned and treated according to the manufacturer's instructions.
We're one of the largest online dental suppliers based on many qualified manufacturers in China.
Any questions, please contact us through eBay message , We will reply within 1-2 business days (public holidays not included).
working time: Monday - Friday 9:00 AM - 5:30PM (Beijing)