-40%
US Automatic ECG Machine 3 channel 12 lead Electrocardiograph EKG Interpretation
$ 189.55
- Description
- Size Guide
Description
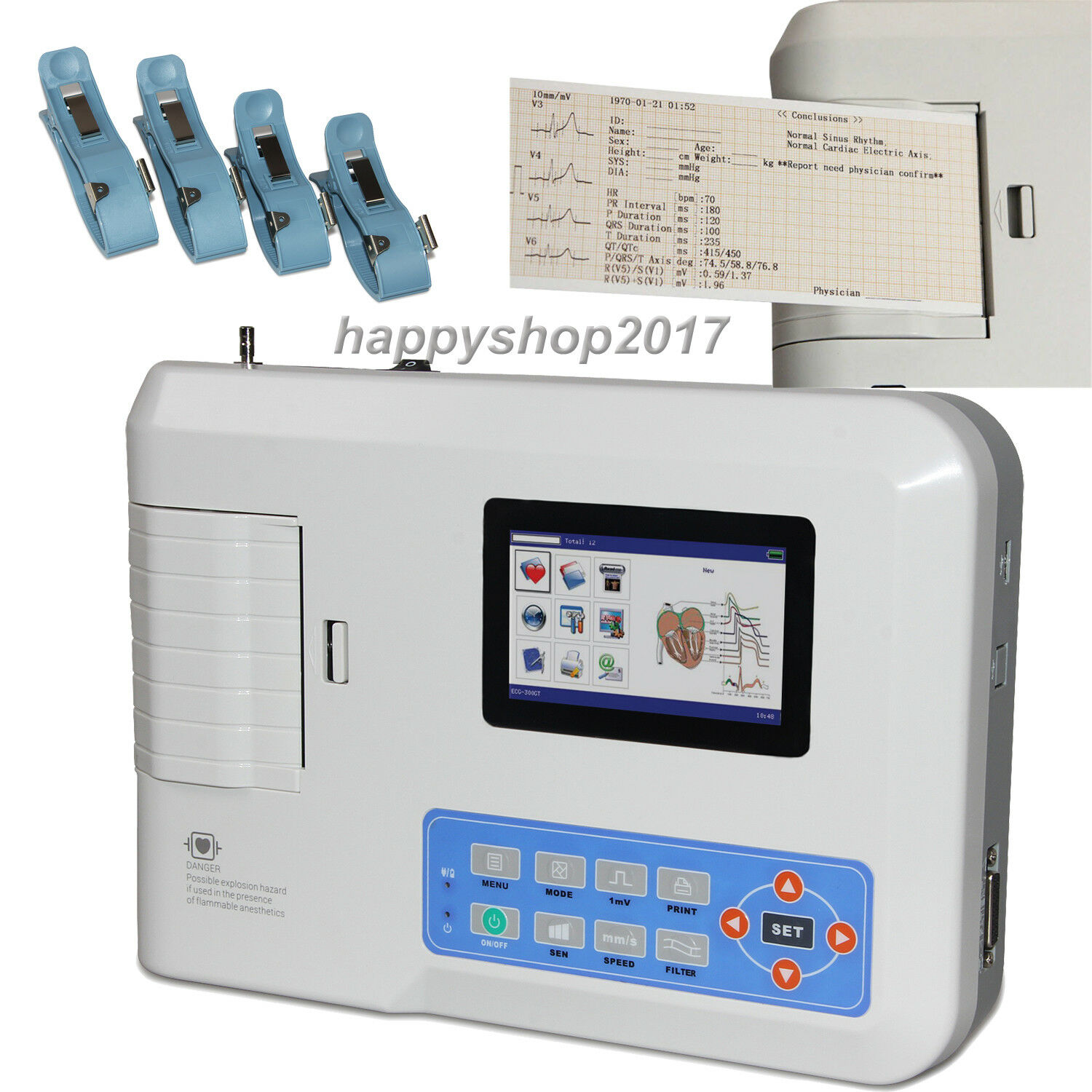
FDA ce ECG300G EKG 3 Channel 12 Lead ECG Machine Electrocardiograph USB PC SoftwareIntroduction
ECG300G is an Electrocardiograph which can collect 12-lead ECG signal simultaneously and print waveform by thermal printing system. It features in, recording and displaying ECG waveform in AUTO/Manual mode, measuring and diagnosing ECG waveform parameters automatically, prompting for “Lead off” and “Lack of paper”, multi-language interface, AC/DC, optional rhythm lead that convenient to observe abnormal heart rhythm, case database management, etc.
Note: The device sold to the countries and regions that have FDA certification requirement does not support automatic analysis and diagnosis function.
Function
1)Sync collection for 12-lead ECG, adopt digital signal processing technology and get high-quality ECG waveform via power frequency filter, baseline filter and EMG filter of ECG signal.
2)Display of 3/6/12-lead ECG, print mode, sensitivity, paper speed and filter state, etc. on one screen, convenient for contrastively diagnosing.
3)Multiply printing modes and formats, including manual, auto 4x3, auto 3x4+1, auto 3x4, auto 2x6+1, auto 2x6, auto 3-2+1, auto 3-2, auto 1x12+1, auto 1x12, rhythm 4, rhythm 3, rhythm 2, etc. Waveform length printed can be adjusted, and with timed print function, which meets different requirements.
4)With the functions of auto-analysis and auto-diagnosis for routine ECG parameters, provide measurement results and auto-diagnosis conclusion for HR, P-R interval, P Duration, QRS Duration, T Duration, Q-T interval, Q-Tc, P Axis, QRS Axis, T Axis, R(V5), S(V1), R(V5)+S(V1), etc. which reduces the doctor’s burden.
5)Build-in large capacity memory stores at least to 1000 cases, which is convenient for doctor to review case and statistic.
6)Multi- language(Chinese, English, Spanish, Turkish, Polish, Italian, French, German, Portuguese, Russian, Ukrainian, Serbian, Vietnamese, Slovenian, Slovak, Bulgarian and Nigerian language) interface and report.
Performance
Input mode: floating and defibrillation protection
Lead: Standard 12-lead
Patient leakage current: <10 µA
Input impedance: ≥2.5MΩ
Frequency response:
Rated input amplitude Input frequency and waveform Relative output response
1.0 0.67Hz~40Hz, Sine wave ±10%a
0.5 40Hz~100Hz, Sine wave +10 %, -30 %a
0.25 100Hz~150Hz, Sine wave +10 %, -30 %a
0.5 150 Hz ~ 500 Hz, Sine wave +10 %, -100 %a
1.5 ≤1Hz,200ms, Triangle wave +0 %, -10 %b
a relative to 10Hz b relative to 200 ms
Time constant:≥ 3.2s
CMRR: >105dB
Filter: power frequency(AC50/60 Hz), EMG(25 Hz/35 Hz (-3 dB)), baseline drift filter
Recording way: thermal printing system
Specification of recording paper: 80 mm(W)×20 m(L) high-speed thermal paper
Time base selection(paper speed): 12.5 mm/s, 25 mm/s, 50 mm/s, error: ±5%
Gain control(sensitivity): 5, 10, 20 mm/mV, accuracy is ±2%; Standard sensitivity: 10 mm/mV±0.2 mm/mV
Recording mode: auto mode, rhythm mode and manual mode
Measurement parameters: HR, P-R interval, P Duration, QRS Duration, T Duration, Q-T interval, Q-Tc, P Axis, QRS Axis, T Axis, R(V5), S(V1), R(V5)+S(V1)
Safety classification: Class I, type CF defibrillation-proof applied part
Polarization resistance voltage: ±610mV
Noise level: ≤12 µVp-p
ECG signal input sampling frequency: 32 kHz
Waveform data processing sampling frequency: 1 kHz
Sampling accuracy: 24-bit
The minimum detection signal: 10 Hz, 20 µV(peak-peak value) deflected sinusoidal signal can be detected
Pacing detection channel: standard II
Accuracy of input signal: ±5%
Amplitude quantization: ≤5µV/LSB
Dimension: 315 mm(L)×215 mm(W)×77 mm(H)
Weight: 1.6 kg
Interchannel time deviation: <100 µs
Power supply:
AC: 100V~240V(50/60Hz)
DC: 7.4V/3500mAh rechargeable lithium battery
Degree of protection against ingress of liquid: IPX0
Working mode: continuous working
Accessories
Standard configuration:
A lead cable
A limb electrode
A chest electrode
A thermal recording paper
A power cord
An earth wire
A user manual
Physical characteristic
Working environment
Temperature: 5℃~40℃
Relative humidity: 25%~95%(non-condensing)
Atmospheric pressure: 700 hPa~1060 hPa
Transport and storage environment
Temperature: -20 ℃~+55 ℃
Relative humidity: ≤95%
Atmospheric pressure: 500 hPa~1060 hPa
Dimension: 315mm(L)×215mm(W)×77mm(H)
Weight: 1.6Kg
Buy Safe Product
The Fingertip Pulse Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG)with the code 197923, and certified by FDA of United States and CE,TUV of Europe. The Fingertip Pulse Oximeter that is FDA 510K Approved
The following FDA Disclaimer is required for all eBay listing in Healthcare category and is included for REFERENCE:
The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If the item is subject to FDA regulation, we will verify your status as an authorized purchaser of this item before shipping of the item.
Shipment
1. We will ship the item within 48 hours after receiving your payment, please kindly leave your detail shipping address and telephone number, both are very important for the shipment,thank you.
2.All items will be shipped to buy's ebay address. Please check your ebay address is right.
3.The item will be declared as lower value for shipping only.We'd like to cooperate with you and declare the value as your request,so that it is easier for you to clear the customs in your country.
4. If you haven't received the item after 2 weeks of your payment, please contact me immediately!
Terms Of Sale
We offer 60 days return policy. 100% satisfaction is our goal!
1.All items are brand new ,with 2 year warranty.
2.If you have a defective item, you want to return or discount. Please contact us within 2 days from you receive the shipment.
3.We will refund the money to you when we get the returned item or make a replacement for you.
4. All returned items must be returned with it's original packaging and accessories. Customer is responsible for shipping charges on returned items.
5.Sometimes there's some delay for the delivery of your package,Please kindly contact us for more information.Please don't open any cases nor return requests on both ebay and paypal,and please don't leave me any negative nor neutral feedback on ebay.I will try my best to give you a satisfied solution.
About Us
CONTEC Medical System-----Focusing on research, manufacture and distribution of medical equipment,
CONTEC Medical System was founded in 1992 as a professional medical equipment producer.
CONTEC was dedicating to research, manufacture and distribution of medical devices, now we have developed more than 20-category products contained Pulse Oximeter,
Sphygmomanometer, ECG, EEG, Ultrasound Equipment, Fetal Doppler,Patient Monitor and Image Equipment,etc
Our products have passed CE, FDA and COS/VIOS certificate. At present, CONTEC produces and distributes more than 2000000 products per year, which were distributed to over 200 countries and regions.
Contact Us
We are Professional Manufacturer--CONTEC MEDICAL SYSTEMS CO.,LTD
Looking forward to establishing a successful business relationship with you.
Address(CN): NO.112 Qinhuang West Avenue,E&T Development zone,Qinhuangdao,China
Address(US):1440 CHASE AVE ,ELK GROVE VILLAGE IL 60007-4825,United States